Large Medical Device Company Accelerated R&D Documentation with Generative AI
Download the PDF to read the full story - Download
Challenge
A leading multinational medical device company serves over 40 million patients annually, delivering life-saving innovations through hundreds of products, and spent around 50% of their R&D budget on documentation ranging from product feature research and risk assessments to manufacturing procedures and distribution guidelines. Despite prior efforts, the documentation process remained inefficient and time-consuming. Quality engineers spent years learning company specific terms and language (to satisfy regulatory and quality requirements) and wasted days locating disjointed information (historical risk and R&D documentation, predicate products, protocols and compliance guidelines, user feedback, complaints), and working through multiple reviews with subject matter experts to improve the documents.
This resulted in weeks of inefficient efforts and intra-engineer variability in output documentation quality and potential for a lower quality medical device that may result in patient harm.
Approach
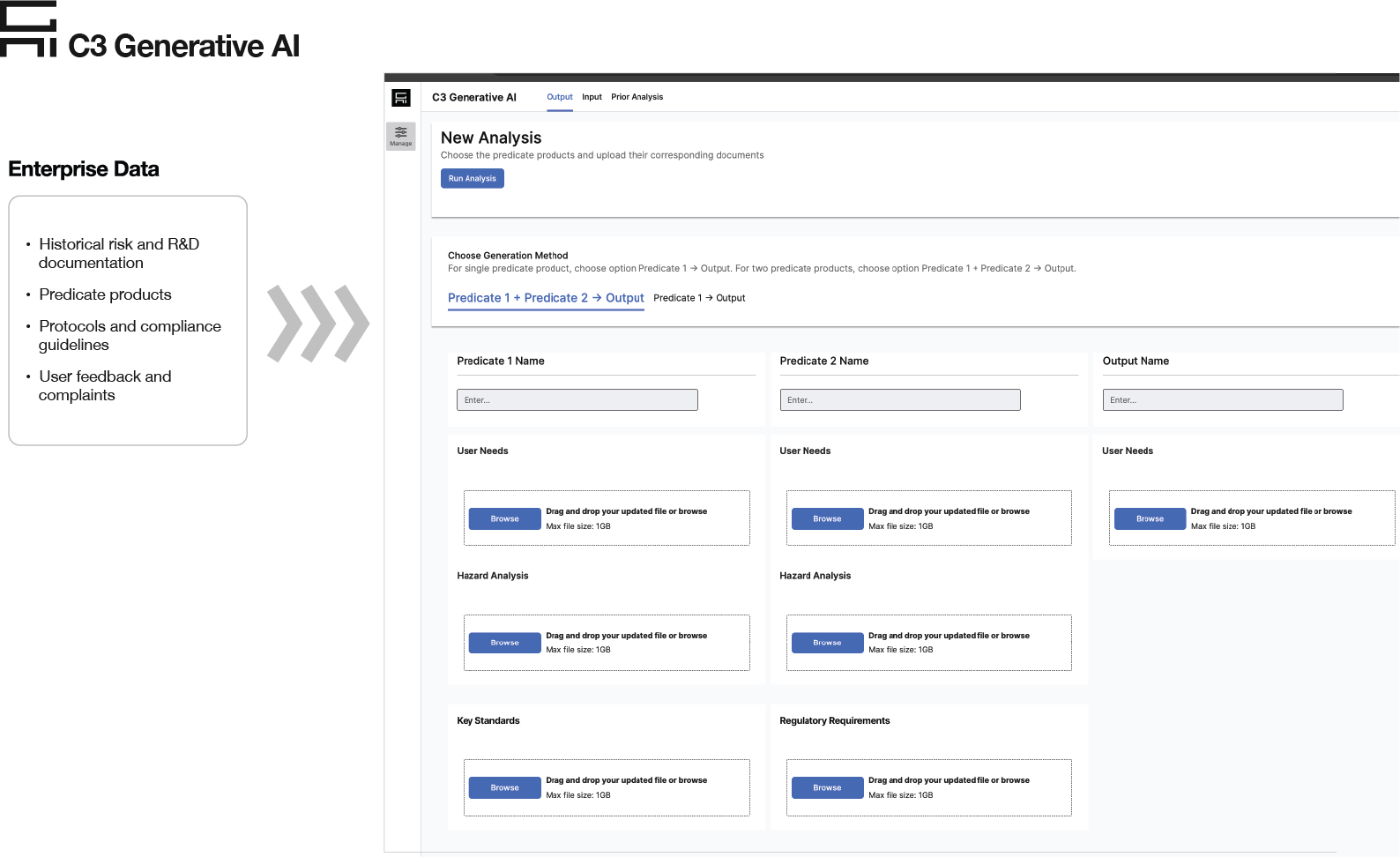
Over 18 weeks, C3 AI configured C3 Generative AI for Regulatory Documentation, focusing first on hazard analysis, one of the most complex and highest importance risk documents, for the Endoscopy division.
A joint team of developers, data scientists and quality engineering subject matter experts identified the corpus of documents required for hazard analysis, the desired output, various input formats, user interfaces and the most efficient workflows. The team designed the approach that incorporated various innovative techniques, such as advanced document processing and chunking to handle non-standard formats and deep learning to inject domain-specific, contextual information. These techniques enabled zero pre-processing work from quality engineers and reliable information retrieval and content generation capabilities that mimic human logics with minimal hallucination.
C3 Generative AI for Regulatory Documentation generated hazard analysis reports in minutes (instead of weeks) ready for quality engineers to review with full output relevance traceability and checking. The application achieved 80-90% accuracy for hazard analysis reports and reduced intra-engineer variability in quality, significantly shortening the documentation time and freeing up quality engineer time for other higher value tasks.”
Project Objectives
- Enable a more streamlined and automatic process for hazard analysis documentation.
- Provide an easy-to-use interface to help QA engineer be more efficient.
- Configure the C3 Generative AI for Documentation Acceleration application that is ready to scale to other use cases across the company.
About the Company
- $15+ billion annual revenue in 2023
- 40+ million patients each year
- $1+ billion R&D investment
Results
Solution Architecture
Proven results in weeks, not years
Take the Next Steps
Learn how our industry-leading Enterprise AI software products can help your organization.
Contact us at IR@C3.ai to learn more about investing at C3 AI.
For all other questions, please contact us here.
